Patients and healthcare providers often ask a question: “Do remote patient monitoring (RPM) devices need FDA approval?” The answer matters more than it seems. Whether a device is cleared or approved by the U.S. Food and Drug Administration (FDA) can directly affect three things:
- Patient safety
- Compliance with regulations
- Clinic reimbursement
But, here’s the key point: when people say “FDA-approved” RPM devices, they’re usually using the wrong term. In reality, most RPM devices are not FDA-approved but FDA-cleared. That distinction, FDA-cleared vs. FDA-approved, confuses many, but it makes all the difference for compliance and reimbursement.
This guide breaks down what “FDA-approved” really means, how to verify devices, and which RPM device categories matter most. By the end, you’ll know exactly how to choose devices that are both safe and compliant for remote patient monitoring programs.
Table of Contents
FDA-Approved vs. FDA-Cleared: What’s the Difference?
The U.S. Food and Drug Administration (FDA) regulates medical devices under a classification system that determines how much review a product needs before it can be sold in the U.S.
This system matters for remote patient monitoring (RPM) because it explains why most devices you see in use are FDA-cleared, not FDA-approved.
Medical Devices Classification by FDA
The FDA uses three main classes:
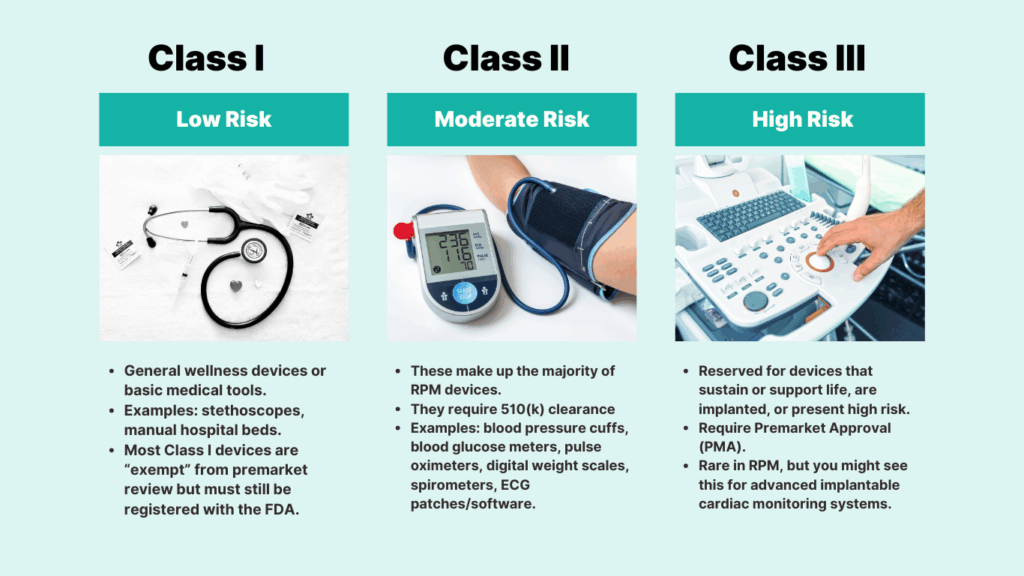
Class I (Low Risk)
These are simple devices with minimal risk to patients. Many are exempt from premarket review, meaning manufacturers must register them with the FDA but do not need to submit safety or effectiveness data. A hospital bed or a manual stethoscope would fit here.
Image Alt Text FDA classification of medical devices
Class I – Low Risk
- General wellness devices or basic medical tools.
- Examples: stethoscopes, manual hospital beds.
- Most Class I devices are “exempt” from premarket review but must still be registered with the FDA.
Class II (Moderate Risk)
This is where most RPM devices belong. Class II devices are subject to more regulatory control because they play a direct role in monitoring health conditions. They typically go through the 510(k) clearance process, which requires manufacturers to prove that their device is “substantially equivalent” to another legally marketed device.
Examples include blood pressure monitors, digital weight scales, pulse oximeters, and glucometers.
Class II – Moderate Risk
These make up the majority of RPM devices.
They require 510(k) clearance
Examples: blood pressure cuffs, blood glucose meters, pulse oximeters, digital weight scales, spirometers, ECG patches/software.
Class III (High Risk)
These are devices that sustain or support life, are implanted, or present significant potential risk to patients. They require Premarket Approval (PMA), the FDA’s most rigorous pathway, which includes scientific evidence and often clinical trial data. While rare in RPM, certain advanced implantable cardiac monitors fall into this category.
Class III – High Risk
Reserved for devices that sustain or support life, are implanted, or present high risk.
Require Premarket Approval (PMA).
Rare in RPM, but you might see this for advanced implantable cardiac monitoring systems.
Difference Between FDA-Approved, Cleared and Registered Devices
With that context, here’s how the FDA describes device status:
FDA-Registered Devices
The manufacturer has listed the device in the FDA’s registration database. This does not mean the FDA has reviewed the product. It is simply a regulatory requirement.
FDA-Cleared Devices (510k)
The FDA has reviewed the device and determined it is safe and effective by comparing it to an existing device. This is the category for the majority of RPM devices. If you are using a remote blood pressure cuff or cellular weight scale, chances are it is 510(k)-cleared.
FDA-Approved (PMA)
The FDA has conducted a full scientific and clinical review of the device. Approval is required for high-risk Class III devices, but it is unusual for standard RPM tools.
FDA Pathways and RPM Devices Overview
| FDA Status | FDA Risk Class | What It Means | RPM Device Examples |
| Registered | Mostly Class I | The company lists devices in the FDA database. No safety review. | General wellness apps, software listings |
| Cleared (510k) | Class II | Device reviewed for safety and effectiveness through “substantial equivalence.” | BP monitors, glucometers, pulse oximeters, digital scales, spirometers, ECG patches |
| Approved (PMA) | Class III | The device undergoes full scientific and clinical trial review. | Implantable cardiac monitoring systems (rare in RPM) |
You can verify any device yourself by searching:
How to Verify a Medical Device in the FDA Database?
By now, you have understood the difference between FDA-cleared and FDA-approved devices. The next step is to check for yourself whether a device is officially recognized by the FDA. The FDA provides public databases where you can do this in just a few minutes. Here’s a step-by-step process to verify a medical device in the FDA database.
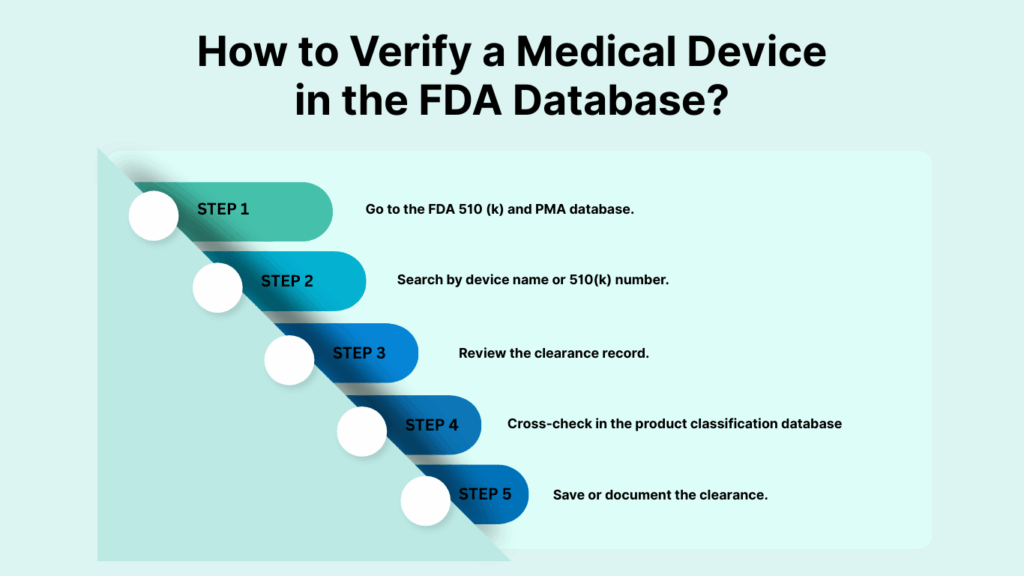
- Go to the FDA 510 (k) and PMA database.
- Search by device name or 510(k) number.
- Review the clearance record.
- Cross-check in the product classification database
- Save or document the clearance.
Here is the detailed overview of each of these steps.
1. Go to the FDA 510(k) and PMA Database
The FDA maintains separate searchable databases for cleared and approved devices:
- 510(k) Premarket Notification Database – for Class II devices that are cleared.
- PMA (Premarket Approval) Database – for Class III devices that are approved.
For most RPM devices (blood pressure monitors, pulse oximeters, glucometers, etc.), you’ll be using the 510(k) database.
2. Search by Device Name or 510(k) Number
If you know the model number or manufacturer, type it into the search bar. If you already have the 510(k) submission number, you can enter that directly.
3. Review the Clearance Record
A typical 510(k) clearance record will show:
- Device Name: the specific model.
- Applicant: the manufacturer.
- Regulation Number: FDA’s regulatory category (e.g., 870.1130 for non-invasive blood pressure monitors).
- Device Class: confirms whether it is Class I, II, or III.
- Decision Date: date the device was cleared.
- Clearance Type: usually “Traditional 510(k).”
This tells you the device has been reviewed by the FDA and is safe for clinical use.
4. Cross-Check in the Product Classification Database
For extra confidence, you can also check the FDA Product Classification Database. Here you can:
- Enter the regulation number (e.g., 870.1130).
- See the device description, risk class, and special controls that apply.
This is helpful if you want to confirm whether the device falls into Class II (most RPM devices) or Class III (rare in RPM).
5. Save or Document the Clearance
Providers should always keep a record (screenshot or PDF) of the clearance. This makes it easier to prove compliance during audits or when billing CMS for remote patient monitoring codes.
Why is FDA Verification Important?
By following this process, providers and clinics can:
- Protect patients by ensuring devices are safe and FDA-reviewed.
- Stay compliant with CMS billing rules, which require devices to meet FDA’s definition of a medical device.
- Avoid reimbursement denials that can happen when using non-cleared consumer gadgets (like fitness trackers or uncertified apps).
List of FDA Approved Medical Devices for Remote Patient Monitoring
When providers or patients search for a “list of FDA approved medical devices,” what they are really asking is: Which devices can I trust for clinical use and reimbursement under remote patient monitoring (RPM)?
The FDA does not publish a single unified list. Instead, each device is documented individually in its database, either as a 510(k)-cleared Class II device or, more rarely, a PMA-approved Class III device.
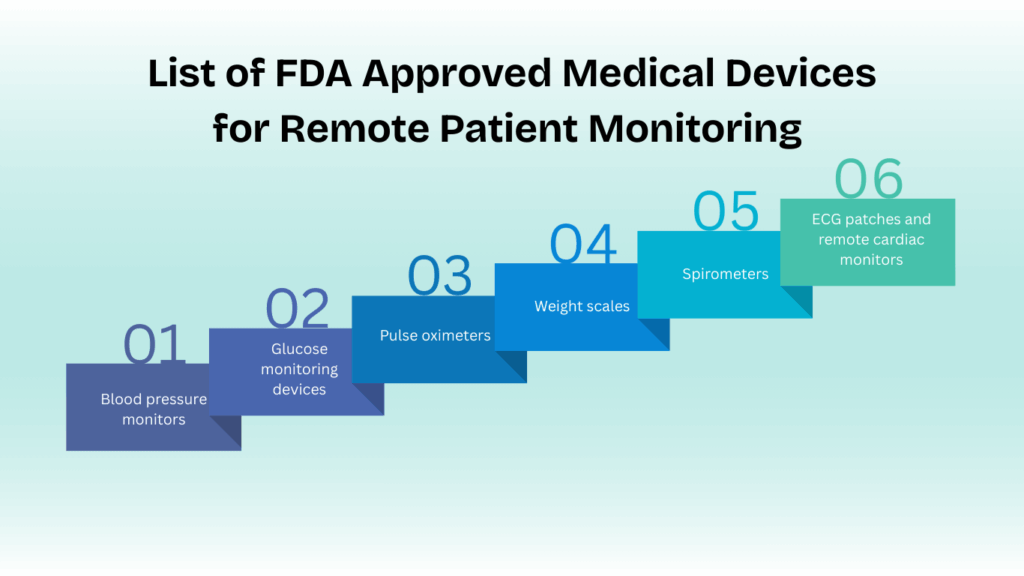
Below is a list of RPM devices used in RPM programs.
- Blood pressure monitors
- Glucose monitoring devices
- Pulse oximeters
- Weight scales
- Spirometers
- ECG patches and remote cardiac monitors
Here is a brief description of each RPM device.
1. Blood Pressure Monitors
Blood pressure monitors are the backbone of most RPM programs, especially for managing hypertension and cardiac conditions. They are almost always Class II devices cleared under the 510(k) process. Devices in this category may connect via Bluetooth or cellular networks, with cellular being particularly valuable for patients who are not comfortable with smartphones.
Platforms like CandiHealth offer preconfigured cellular devices ready to use out of the box. Want to know more about it? Book your free demo now.
2. Glucose Monitoring Devices
For diabetes management, glucose monitors are essential. These devices automatically transmit readings, usually via Bluetooth to an app or cellular hub, ensuring timely data delivery to providers. Their clearance guarantees they meet FDA standards for accuracy and safety, making them compliant for clinical use.
3. Pulse Oximeters
Pulse oximeters measure oxygen saturation and heart rate, critical for managing COPD, asthma, and post-COVID conditions. Most pulse oximeters in RPM programs are Class II devices, often using Bluetooth connectivity to sync data. Their clearance ensures providers can rely on accurate, clinical-grade readings.
4. Weight Scales
For congestive heart failure patients, small weight changes can indicate fluid buildup. That’s why cellular-enabled weight scales are a core RPM tool. Unlike consumer smart scales, these devices are purpose-built for clinical monitoring and integrate easily with RPM platforms.
5. Spirometers
Spirometers measure lung function and are particularly useful in monitoring COPD and asthma patients remotely. These devices typically use Bluetooth for data transmission and are reviewed under the Class II clearance pathway.
6. ECG Patches and Remote Cardiac Monitors
ECG patches are increasingly common in RPM programs to detect arrhythmias and support cardiology care. Devices in this category provide continuous cardiac monitoring, often uploading data via apps or cellular hubs. Their clearance confirms clinical-grade reliability, which is essential for reimbursement and patient safety.
CMS Requirements for FDA-Approved Remote Patient Monitoring Devices
The Centers for Medicare & Medicaid Services (CMS) has made it clear that RPM devices must meet the FDA’s definition of a medical device. In other words, they cannot simply be consumer wellness gadgets or fitness trackers. Devices must be able to automatically record and digitally transmit physiological data. Manual logging by patients does not count.
The second critical requirement is the “16 readings in 30 days” rule. For CPT codes 99453 (setup and patient education) and 99454 (supply of device and data transmission), the device must collect and transmit at least 16 days’ worth of measurements during a 30-day billing cycle.
If fewer than 16 readings are recorded, reimbursement for these codes is not allowed. Importantly, this requirement applies only to the device supply codes, not to the treatment management codes (99457 and 99458).
To make this easier for providers, here’s a quick RPM devices compliance checklist:
- The device must qualify as an FDA medical device.
- Data must be automatically recorded and digitally transmitted (not patient self-reported).
- 16 of 30 days required for billing CPT 99453 and 99454.
- No 16-day requirement for CPT 99457 and 99458 (treatment management codes).
- Always document FDA clearance/approval number (510(k) or PMA) in vendor records.
Want to know more about RPM billing guidelines? Read the following blog: RPM Billing Guidelines 2025: CPT Codes & Compliance Tips
Final Thoughts
Most remote patient monitoring devices aren’t FDA-approved — they’re FDA-cleared through the 510(k) process. This distinction matters because only FDA-recognized devices meet CMS requirements for reimbursement and ensure patient safety. Before launching or expanding an RPM program, always verify devices in the FDA database and make sure they meet the 16-day data rule for compliance.
At CandiHealth, we take the guesswork out of this process. Our RPM platform comes preconfigured with FDA-cleared devices, cellular connectivity, and a centralized dashboard, so clinics can stay compliant while focusing on patient care.
Get started with CandiHealth today and build your RPM program on devices you can trust.
FAQs About FDA-Approved Remote Patient Monitoring Devices
Is remote patient monitoring FDA approved?
Remote patient monitoring itself is not FDA-approved because it’s a care model, not a device. What is regulated are the devices used in RPM programs. Most of these devices, like blood pressure monitors or glucometers, are FDA-cleared through the 510(k) process rather than FDA-approved.
Which device is commonly used in remote patient monitoring?
The most commonly used RPM devices are blood pressure monitors, glucose meters, pulse oximeters, digital weight scales, and ECG patches. These tools track vital signs that are crucial for managing chronic conditions like hypertension, diabetes, COPD, and heart failure. Most of them are Class II medical devices that are FDA-cleared.
Do wearable devices need FDA approval?
Yes, if they are used for clinical purposes such as diagnosing or monitoring a medical condition. Wearables that track steps or general wellness metrics do not need FDA approval or clearance. But wearables marketed as medical devices (like ECG patches or continuous glucose monitors) must be either FDA-cleared or FDA-approved depending on their risk class.
How to find if a device is FDA approved?
You can verify any device directly in the FDA’s databases. Search the 510(k) Database for Class II cleared devices or the PMA Database for Class III approved devices. Enter the device name, manufacturer, or clearance number to confirm its FDA status.